ratings
In this course, we will discuss the complete atomic structure chapter 2 of chemistry class 11. This chapter will be discussed in detail from CBSE, IIT-JEE (Mains and Advanced), and NEET point of view.
1 week, 3 days
June 1, 2021
10
14 hours
Key notes of the chapter are:
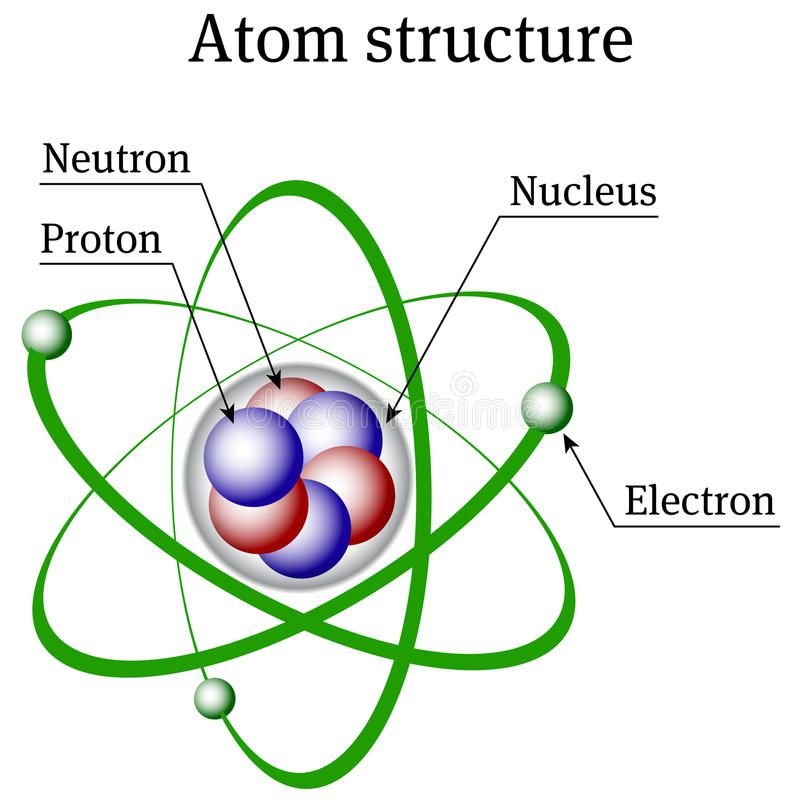
Aoms:
- Atom is the smallest particle of matter that can take part in a chemical reaction.
- Atom is made of electron, proton and neutrons.
- Atom is not capable of independent existence.
- Two or more atoms combine together to form molecules.
Electron:
- Negatively charged particle discovered on the basis of ‘cathode ray discharge tube’ experiments.
- Conclusion from ‘cathode ray discharge tube’ experiment:
Cathode rays start from cathode and move toward anode.
These rays are not visible but their behaviour can be observed with fluorescent or phosphorus sent material.
In the absence of electric or magnetic field these travels in strait lines.
In the presence of electric or magnetic field the behaviour of cathode rays is similar to negatively charged particles which suggest that these rays contain negatively charge particles called electron.
Proton:
- Positively charged particle discovered on the basis of anode ray experiment.
- Some of the characteristics of anode rays, also called canal rays, are:
These travel in straight line and posses mass many times the mass of an electron.
These are not originated from anode.
These are deflected by electric and magnetic field.
Unlike cathode rays, the positively charged particles depend upon the nature of the gas from which these originate.
Neutron:
- Neutral particles discovered by bombarding a thin sheet of beryllium by α- particles.
- Conclusion from α- particles scattering experiment:
Most of the α-particles passed through foil undeflected, indicating most of the space in atom is empty.
Some of the α-particles are deflected to certain angles, which means that there is positively mass present in atom.
Only some of the α-particles suffered large deflections, which means that the positively charged mass must be occupying very small space.
Strong deflections or even bouncing back of α-particles from metal foil indicate the direct collision with positively charged mass in atom.
Thomson model of atom:
- atom is considered asa uniform positively charged sphere with radius about 10-10 m, in which electrons are is uniformly distributed.
- Electrons are embedded in such a manner to give most stable electrostatic arrangement.
- Mass of atom is assumed to be uniformly distributed in atom.
- Also known as plum pudding raisin pudding orwatermelon model.
Rutherford’s Nuclear Model of Atom:
- Based upon α-particles scattering experiment.
- Most part of the atom is empty.
- Atom possesses a highly dense, positively charged centre, called nucleus of the order 10-13 cm.
- Entire mass of the atom is concentrated inside the nucleus.
- Electrons revolve around the nucleus in circular orbits.
- Electrons and the nucleus are held together by electrostatic forces of attraction.
Drawbacks of Rutherford’s Model:
- It doesn’t explain the stability of atom.
- It doesn’t say anything about the electronic distribution of electrons around nucleus.
Answer the following questions to check your preparedness:
Q. What information is obtained from the cathode rays experiment about the structure of atom?
Q. How was the presence of proton detected and what are their characteristic?
Q. Give the characteristic properties of alpha (a) rays.
Q. Discuss the weakness of Rutherford atomic model.
Q. Calculate the mass and charge of one mole of electrons.
Atomic Number:
- Equal to the total number protons present in the nucleus or total number of electrons present the neutral atom.
- Represented by Z.
Mass Number:
- Mass number of an element = number of protons + number of neutrons
- Denoted by A.
Isotopes:
- These are the atoms of the same element having the same atomic number but different mass number.
- For example: 1H1,1H2,1H3
Isobars:
- These are the atoms of different elements having the same mass number but different atomic number.
- For example: 18Ar40 , 20Ca40
Electromagnetic radiations:
- Radiations associated with electrical and magnetic fields.
- When an electrically charged particle moves under acceleration, alternating electrical and magnetic fields are produced and transmitted in the form of waves called electromagnetic waves or electromagnetic radiations.
Properties of electromagnetic radiations:
- Oscillating electric and magnetic field are produced by oscillating charged particles. These fields are perpendicular to each other and both are perpendicular to the direction of propagation of the wave.
- These waves do not require medium i.e. electromagnetic wave can travel in vacuum.
Characteristics of electromagnetic radiations:
- Wavelength: It may be defined as the distance between two neighbouring crests or troughs of wave. It is denoted by λ.
- Frequency (ν): It may be defined as the number of waves which pass through a particular point in one second.
- Velocity (v): It is defined as the distance travelled by a wave in one second. In vacuum all types of electromagnetic radiations travel with thesame velocity which is 3 ×10 8m/sec. It is denoted by v.
- Wave number:Wave number is defined as the number of wavelengths per unit length. Velocity = frequency × wavelength, i.e, c = νλ
- Amplitude (a): It is the height of the crest or depth of the trough of a wave.
Particle nature of electromagnetic radiation (Planck's Quantum Theory):
- The radiant energy is emitted or absorbed not continuously but discontinuously in the form of small discrete packets of energy called ‘quantum’. In case of light, the quantum of energy is called a ‘photon’
- The energy of each quantum is directly proportional to the frequency of the radiation, i.e. E α υ
Or E = hυ
Where h = Planck’s constant = 6.626 × 10-27 Js
- Energy is always emitted or absorbed as integral multiple of this quantum, i.e,
E = nhυ
Where n =1,2,3,4,.....
Black body:
- An ideal body, which emits and absorbs all frequencies, is calleda black body.
- The radiation emitted by such a body is called black body radiation.
Photoelectric effect:
- The phenomenon of ejection of electrons from the surface of metal when light of suitable frequency strikes it is calledphotoelectric effect. The ejected electrons are called photoelectrons.
- Number of electron ejected is directly proportional to intensity (or brightness) of light.
- There is characteristic minimum frequency (ν0 threshold frequency) below which photoelectric effect is not observed.
- If ν > ν0 then electrons comes out with kinetic energy which increases with increase in frequency of light.
- Kinetic energy of ejected electrons is given by-
h ν = h ν0+ ½(meV2)
Dual behavior of electromagnetic radiation:
- The light possesses both particle and wave like properties.
- whenever radiation interacts with matter, it displays particle like properties (Black body radiation and photoelectric effect).
- When it propagates, it shows wave like properties (interference and diffraction).
Spectrum:
When a white light is passed through a prism, it splits into a series of coloured bands known as spectrum.
Types of spectrum:
It is of two types:
(a) Continuous and line spectrum: The spectrum which consists of all the wavelengths is called continuous spectrum.
(b) Line spectrum: A spectrum in which only specific wavelengths are present is known as a line spectrum.
Electromagnetic spectrum:
- It is a continuous spectrum.
- It consists of a range of electromagnetic radiations arranged in the order of increasing wavelengths or decreasing frequencies.
Spectrum can also be classified as follows:
- Emission spectrum: The spectrum of radiation emitted by a substance that has absorbed energy is called an emission spectrum.
- Absorption spectrum: It is the spectrum of radiation transmitted through a substance, showing dark lines or bands due to absorption at specific wavelengths.
Try following questions to check your preparedness:
Q. Define spectrum and discuss line spectrum.
Q. Calculate the wavelength, frequency and wavenumber of a light wave whose period is 2.0 × 10–10 s.
Q. Mass of an electron is 9.1 × 10-31 kg. if its K.E. is 3.0 × 10-25 J, calculate its wavelength.
Q. Calculate the wave number of lines having the frequency of 5 x1016 cycles per second.
Studens
About Instructor
More Courses by Insturctor
Course Currilcum
-
- Lecture 1 FREE 00:30:00
-
- Lecture 1 01:30:00
- Lecture 2 01:30:00
- Lecture 1 01:30:00
- Lecture 2 01:30:00
- Lecture 3 01:30:00