Cahn Ingold Prelog (CIP) Rules, Assigning R and S Configurations
1. Chiral Centers And The Problem Of Naming
Cahn ingold Prelog Rules
Enantiomers: molecules that are non-superimposable mirror images of each other. Perhaps the most memorable example is these “enantiocats”

Each of these cats is said to be “chiral”: they lack a plane of symmetry.
What causes molecules to have chirality ?
The most common source of chirality is a “chiral centre”: typically a tetrahedral carbon attached to four different “groups”, or “substituents”. For each chiral centre there are two (and only two!) different ways of arranging the 4 different substituents, which gives rise to two different configurations.
The purpose of this post is to introduce and describe the nomenclature we use to describe these configurations: the (R)/(S) notation, or Cahn-Ingold Prelog Rules.
Let’s look at a simple example.
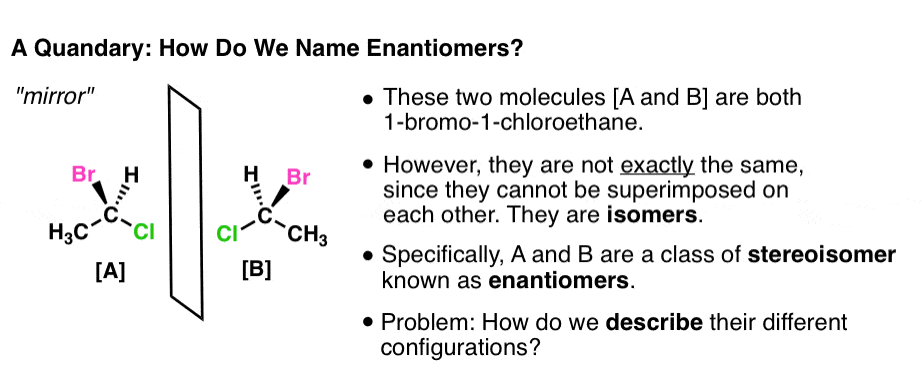
Both of these molecules are 1-Bromo-1-chloroethane. But they are not exactly the same molecule, in the same way, that your left shoe is not exactly the same as your right. They are non-superimposable mirror images of each other. How do we communicate this difference?
One way would be to describe their physical properties. For example, although these two molecules have the same boiling point, melting point, and share many other physical properties, they rotate plane-polarized light in equal and opposite directions, a property called optical rotation. We could use (+)-1-bromo-1-chloroethane to refer to the isomer that rotates polarized light to the right (clockwise, or “dextrorotatory”) and use (-)-1-Bromo-1-chloroethane to refer to the isomer that rotates polarized light to the left (counterclockwise, or “levorotatory”).
However, this nomenclature suffers from a serious problem. There is no simple correlation between the arrangement of substituents around a chiral center and the direction in which polarized light is rotated. Another solution is needed.
2. The Cahn Ingold Prelog (CIP) System For Naming Chiral Centers
A solution to this quandary was proposed by Robert Cahn, Chris Ingold, and Vladimir Prelog in 1966. The resulting “CIP” protocol works as follows:
Coordination Compounds
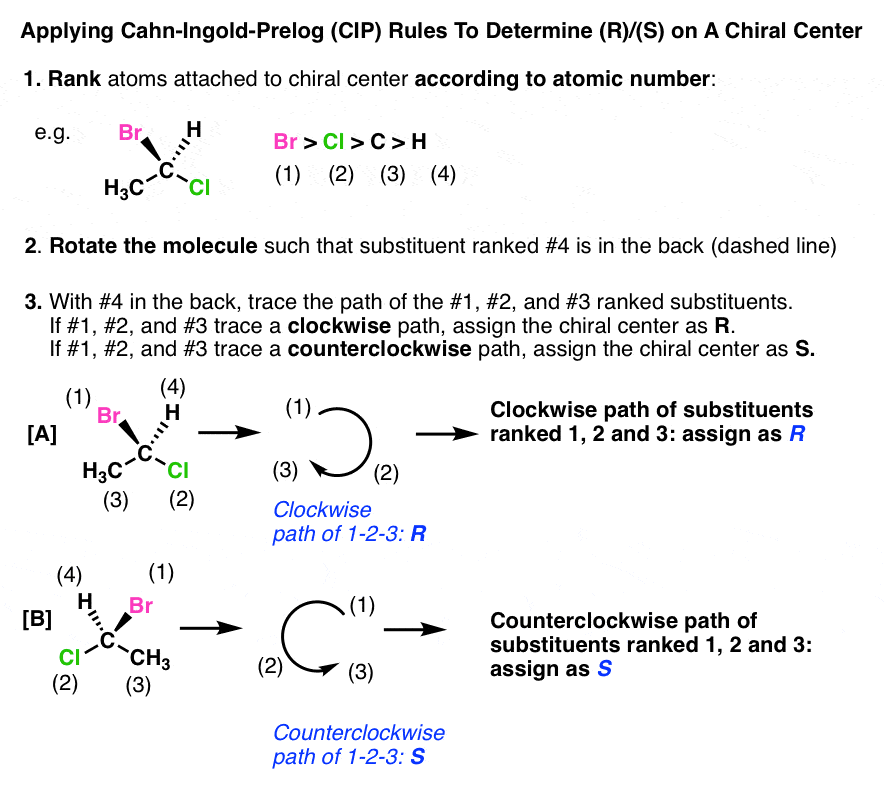
- 1. Prioritize the four groups around a chiral center according to atomic number. The highest atomic number is assigned priority #1, and the lowest atomic number is assigned priority #4.
- 2. Orient the chiral centre such that the #4 priority substituent is pointing away from the viewer. For our purposes, it’s enough for it merely to be attached to a “dashed” bond.
- If the path traced from 1-2-3 is clockwise, the chiral center is assigned (R) (from Latin, rectus)
- If the path traced is counter clockwise, the chiral center is assigned (S) (from the Latin sinister)
- Now we have a better way to describe molecules [A] and [B] shown above. Molecule [A] is named (R)-1-bromo-1-chloroethane, and molecule [B] is named (S)-1-bromo-1-chloroethane:
We should reiterate that the designations (R) and (S) bear no relationship to whether a molecule rotates plane-polarized light clockwise (+) or counterclockwise (-). For example, the most common naturally occurring configuration of the amino acid alanine is (S), but its optical rotation (in aqueous acid solution) is (+).
3. What About When #4 Is Not In The Back?
That seems simple enough! “Is that it?”, you might ask.
Uh, no. As it happens, there’s a few bumps in the road toward determining (R)/(S) once we get beyond the simple example above.
These “trickier cases” fall into three main categories.
- 1. What if the #4 substituent is not helpfully pointing away from the viewer, as it was in our example above? What if it’s in the “front” (i.e. attached to a “wedged” bond) or, heaven forbid, in the plane of the page?
- 2. Assigning priorities in complex situations. What do we do in situations where a chiral centre has two or more identical atoms attached? In other words, how do we break ties?
- 3. What do we do if the molecule is drawn a peculiar way, such as in Fischer or Newman projections?
We’re not going to be able to fully address all of these issues in this post. But we can certainly deal with #1 and make some headway with #2. We’ll deal with #3 in a future post.
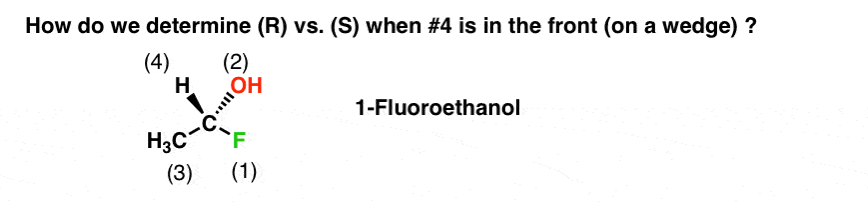
4. Determining R/S When The #4 Substituent Is In Front (i.e. on a “Wedge”): A Short Cut
Let’s first consider the molecule below. The name of this molecule is (R)-1-fluoroethanol. It is listed below with priorities assigned based on atomic number. In this case F>O>C>H. So F is #1 and H is #4. The tricky part here is that the #4 priority is pointing out of the page (on a “wedge”).
How do we determine (R)/(S) in this case? There are two ways to do it.
Many instructors will tell you to “simply” rotate the molecule in your head so that the #4 priority is on a dash. Then you can assign R or S in the traditional way. This “simple” advice is not always an easy task for beginners.
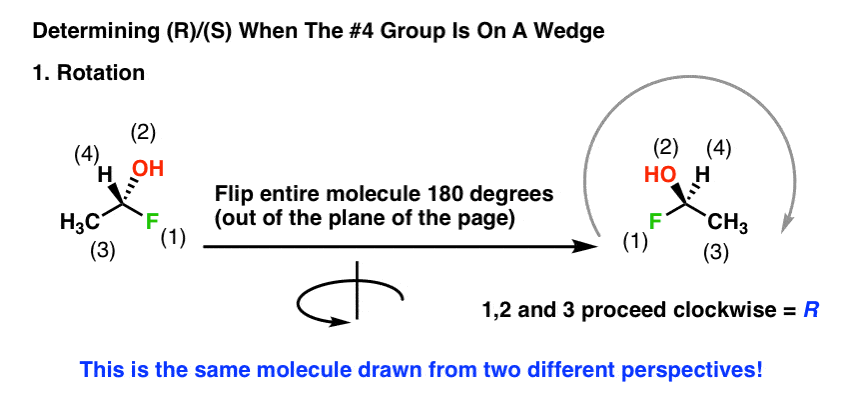
Thankfully, it is technically unnecessary to perform such a mental rotation.
Here’s a way around this. When the #4 priority is on a wedge you can just reverse the rules. So now we have two sets of rules:
If the #4 priority is on a dash:
- Clockwise = R
- Counterclockwise = S
If the #4 priority is on a wedge, reverse the typical rules:
- Clockwise = S
- Counterclockwise = R

R and S can easily be assigned to either picture of the molecule. I still encourage you to use a model kit and learn how to do so, however. Organic chemistry is much easier to understand, and much more beautiful, if you can master how to visualize a tetrahedral carbon atom.
Coordination Compounds
5. Determining R/S When The #4 Group Is In The Plane Of The Page?
What if the #4 priority is in the plane of the paper, for example on a line? In this case it’s impossible to assign R and S in the traditional way. You’d have a 50:50 shot of getting it correct: not good odds. Again, if you can redraw the molecule in your head, then it’s better to just do that. If you can’t do this reliably then you need to learn the “single swap” concept.
Here’s how it works. Swapping any two groups on a chiral centre will flip the configuration of the chiral centre from R to S (and vice versa).
Knowing this, we can do a nifty trick.
- Take the #4 substituent (in the plane of the page) and swap it with the substituent in the back [If the configuration is R, this will switch it to S. If the configuration is S, this will flip it to R. We’ll need to account for this in step #3].
- Redraw the chiral centre, and determine R/S on the new chiral centre which now has group #4 in the back.
- Whatever result you got, flip it to its opposite to account for the fact that you did a single swap in step #1.
Here’s an example. Note that here I first switched #4 and #3, but the main point is to switch two groups so that #4 is out of the plane of the paper.

This method always works, assuming you’ve determined the four priorities accurately. (It also works for cases when #4 is on a wedge).
However, sometimes we’re not in the position of dealing with 4 different atoms attached to a chiral carbon. For instance, it’s possible to have chiral carbons which are attached to 4 carbons. So how do we break the ties in these cases?
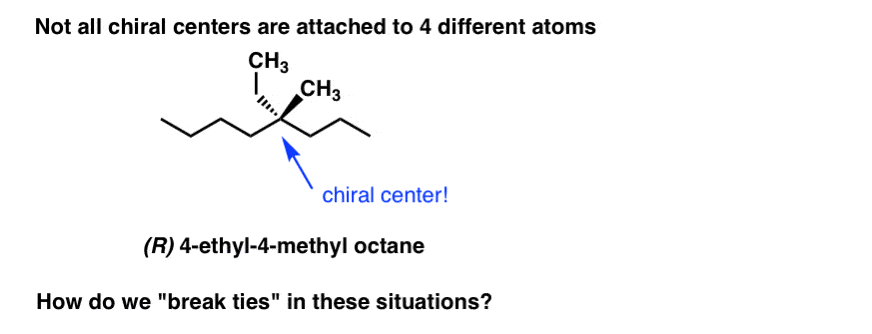
6. Determining CIP Priorities: Breaking “Ties” With The “Dot Technique”
The quick answer is to use the “dot technique”. Here’s how it works. Let’s do it for 4-ethyl-4-methyloctane, above.
- 1. Go outward from the chiral centre to each of the surrounding 4 atoms and assign priorities (based on atomic number) to each of these atoms.
- [Sometimes it’s helpful to draw dots on each of these atoms.]

2. If there is a tie, list the atoms attached to each of those dotted atoms in decreasing order of atomic number.

3. Compare each list, atom by atom. In our example, since C>H, (C,H,H) takes priority over (H,H,H) so the CH3 group is assigned priority #4.

4. If there is still a tie, move the dots to the highest ranking atom in the list (i.e. the atom with highest atomic number). The dots are helpful because they help you to keep track of where you are, which can be important in complex examples.

5. In this case, we keep moving along the chain. By the way, if you ever reach the end of the chain without determining a difference, that means that the groups are identical and it isn’t a chiral centre after all.

6. By this point we have enough information to assign (R)/(S). Since priority #4 is in the front, we can also break out our “opposite rule” for good measure:

Coordination Compounds
(Advanced) References and Further Reading
- 1. Specification of Molecular Chirality
R. S. Cahn, Sir Christopher Ingold, V. Prelog
Angew. Chem. Int. Ed. 1966, 5 (4), 385-415
DOI: 10.1002/anie.196603851
This is not the first paper on the topic by the authors (see Refs. 4 and 5), but it is a major publication and an attempt to consolidate all the information on chirality in a single place. This paper discusses the various types of chirality possible in chemistry (not just at tetrahedral carbons!) and how to assign chirality unambiguously.
- 2. Basic Principles of the CIP‐System and Proposals for a Revision
Dr. Vladlmir Prelog and Prof. Dr. Günter Helmchen
Angew. Chem. Int. Ed. 1982, 21 (8), 567-583
DOI: 10.1002/anie.198205671
An update to Ref. #1, which addresses a lot of edge cases that may come up in complex stereochemical assignments. - 3. CHIRALITY IN CHEMISTRY
Vladimir Prelog
Nobel Lecture, 1975
https://www.nobelprize.org/prizes/chemistry/1975/prelog/lecture/
Prelog’s Nobel Lecture. Nobel Lectures are fascinating to read as they give insight into the life of scientists and the path to discovery, which is rarely linear.
- 4. “Absolutely” simple stereochemistry
Philip S. Beauchamp
Journal of Chemical Education 1984, 61 (8), 666
DOI: 10.1021/ed061p666
This paper describes a simple method for determining stereochemistry of tetrahedral carbons using the hands, suitable for undergraduate students of organic chemistry.
- 5. A simple hand method for Cahn-Ingold-Prelog assignment of R and S configuration to chiral carbons
Martin P. Aalund and James A. Pincock
Journal of Chemical Education 1986, 63 (7), 600
DOI: 10.1021/ed063p600
A follow-up to the previous paper (Ref #4), but sadly it is incomplete! - 6. A Web-Based Stereochemistry Tool to Improve Students’ Ability to Draw Newman Projections and Chair Conformations and Assign R/S Labels
Nimesh Mistry, Ravi Singh, and Jamie Ridley
Journal of Chemical Education 2020, 97 (4), 1157-1161
DOI: 10.1021/acs.jchemed.9b00688
This paper discusses a web-based tool that helps students with visualization of chiral compounds and assignment of stereochemistry as per the Cahn-Ingold-Prelog (CIL) rules. See ref. 34 in the paper for the link.
Coordination Compounds
₹999.00Original price was: ₹999.00.₹299.00Current price is: ₹299.00. per 60 daysRespiration in Plants
₹1,999.00Original price was: ₹1,999.00.₹999.00Current price is: ₹999.00. per 60 daysAnimal Kingdom Biology Class 11
₹2,499.00Original price was: ₹2,499.00.₹999.00Current price is: ₹999.00. per 60 daysSulphuric Acid 10 ICSE 2022
Acid Bases SaltsChemical bonding Class 10 ICSEclass 10 ICSESulphuric Acid 10 ICSE 2022
FREE
Laws of Motion NLM Physics Class 11
Laws of Motion NLM Physics Class 11For demo lectures contact us at 9+91041004111
₹2,499.00Original price was: ₹2,499.00.₹249.00Current price is: ₹249.00. per 60 daysBiomolecules Chemistry Class 12 Term 1 MCQ based
chemistry class 12Class XIIOrganic ChemistryBiomolecules Chemistry Class 12 Term 1 CBSE MCQ based For demo lectures contact us at...
₹999.00Original price was: ₹999.00.₹199.00Current price is: ₹199.00. per 60 days
TYPE YOUR QUESTION & GET VIDEO SOLUTIONS
DOCTOR LOGICS POWERED SEARCH
DOCTOR LOGICS ADVANTAGES
Complete Solution to your School and Competitive Exams in Hinglish Language
At Doctor Logics (Sunny Garg Institute of Competitions) you will find more than 1000 Video Tutorials, as part of Subject Courses, prepared by IITian Faculties of Kota experience. These Tutorials give students a highly engaging & personal teaching experience. Students also get access to top-notch Study Material like NCERT book solutions and chapter/revision notes. They can practice to perfection via advanced Test Series and ask their Doubts anytime from our Experts.
Students can opt for Doctor Logics (Sunny Garg Institute of Competitions) revolutionary LIVE Online Tutoring that combines all the above advantages with a Master online teacher to take care of LIVE Class interaction. Teachers use highly effective, well-researched, and scalable Doctor Logics Hybrid Teaching Methodology to ensure the highest standards of teaching consistently in all the LIVE Classes!
With Doctor Logics (Sunny Garg Institute of Competitions) you can prepare for:
- School Education (Class 8 to 12)
- IIT JEE Mains & Advanced
- NEET